 |
The cover presents the tuning of the interfacial hydrogen source for acidic CO₂ reduction. A dynamic azole layer on bismuth catalysts disrupts the water hydrogen-bond network, hindering Grotthuss proton transport to suppress hydrogen evolution. This opens a selective route for converting CO₂ into formic acid with significantly enhanced efficiency. |
 |
The cover image highlights electron transfer from Si–ONa–Al moieties to iron active sites in the Fe/ZSM-5-0.25Na drives selective C–C coupling between methane and CO, efficiently steering the reaction toward acetic acid formation. In their Research Article, authors demonstrate a strategy bypasses traditional Brønsted acid dependency, employs metal-electronic modulation to enhance catalytic efficiency, and establishes an innovative pathway for methane conversion. |
 |
The cover presents a K-FeMn/Hollow ZSM-5 composite tandem catalyst capable of achieving efficient one-step conversion of CO₂ to para-xylene. The Hollow ZSM-5 component enhances selective para-xylene diffusion due to its unique topology structure, while its passivated external Brønsted acid sites suppress undesired isomerization. This design markedly improves the para-xylene space-time yield, offering a promising pathway for direct CO₂ upcycling. |
 |
The cover depicts the CO oxidation on amorphous CeOₓ nanoislands and crystalline CeOₓ nanoislands. CeOₓ nanoclusters with varying degrees of crystallinity are synthesized through a calcination-treatment protocol. Lattice oxygen in crystalline CeOₓ nanoclusters facilitates the efficient transformation of CO molecules adsorbed on Pt clusters, whereas oxygen in amorphous CeOₓ nanoclusters requires overcoming a significant energy barrier to react with CO. |
 |
The cover depicts the structure of a Pt/CeOₓ/SiO₂ catalyst featured by the atom-precise low-nuclearity Pt clusters on CeOₓ nanoisland nests. The clusters maintained their nuclearity and were resistant to sintering in H₂ at temperatures of ≤600 °C and atmospheric pressure. Catalysis tests demonstrate that these Pt clusters are more active than mononuclear Pt, also exhibiting higher steady-state activity than larger and smaller platinum clusters for ethylene hydrogenation. |
 |
The cover depicts a future scenario of human settlement on Mars, where energy is supplied by solar power and synthesizing liquid products through CO₂ hydrogenation. It highlights the pivotal role of CO₂ hydrogenation to liquid products in sustainable resource utilization for deep-space exploration and extraterrestrial settlement. |
 |
On the cover, the surface Pt-Ti alloyed layers on Pt particles supported on TiO₂ exhibit excellent activity in catalyzing the conversion of CH₄, CO, and O₂ into CH₃OH, HCHO, and HCOOH. This work reveals that CO promotes the dissociation of O₂ on the surface Pt-Ti alloyed layers, subsequently activating CH₄ to form oxygenates. |
 |
The cover illustrated thio ligand-modified Au nanoparticles as an extraordinary electrocatalyst to enhance the electroreduction of nitrate to ammonia due to the regulated electronic structure. |
 |
The cover shows the structure of Cox+–Co0 interfacial sites which promote propene hydroformylation. Compared with the surface of metallic Co (111), the Cox+–Co0 interfacial sites work synergistically and lower the energy barrier of propene hydroformylation. |
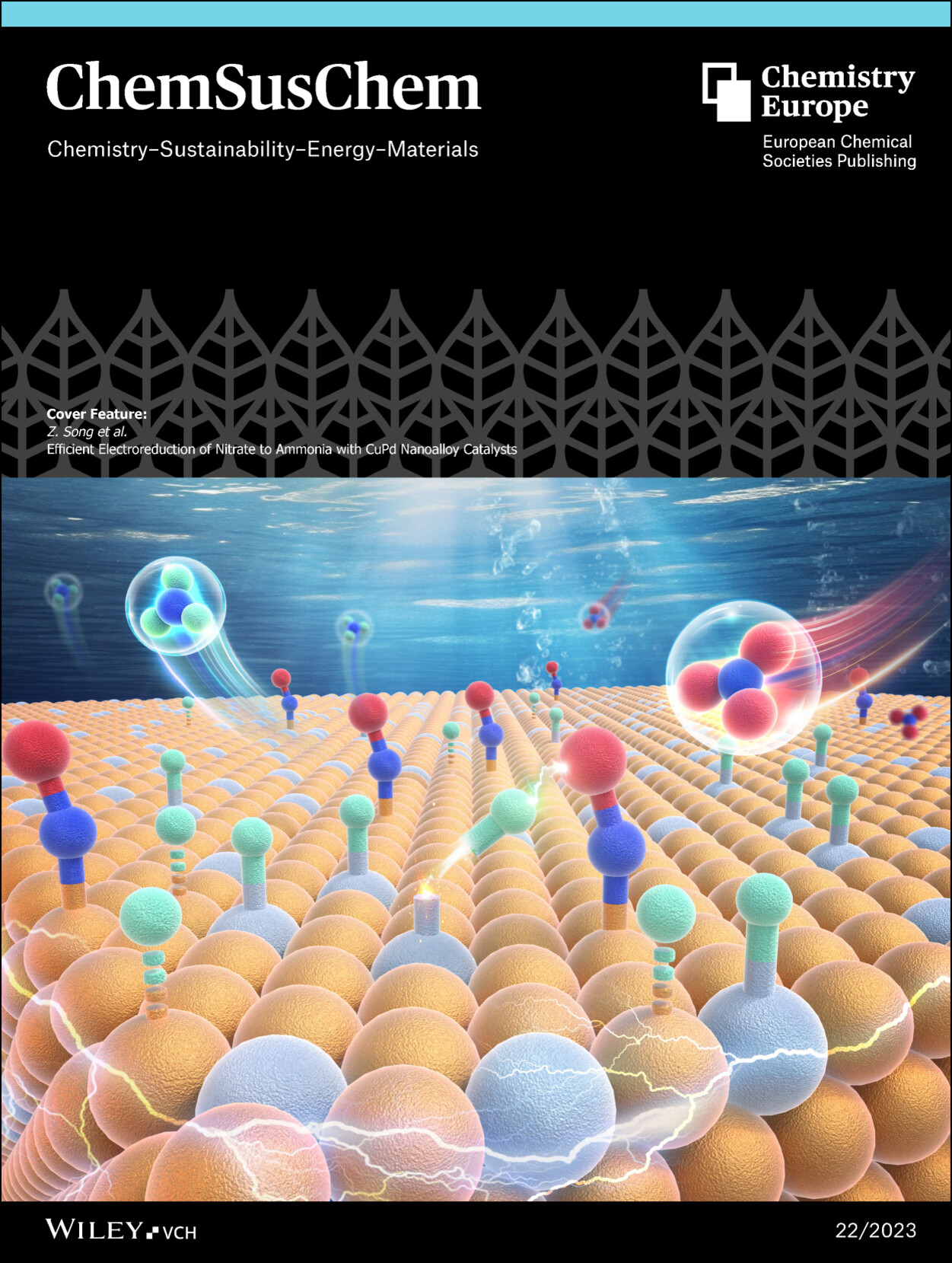 |
The Cover Feature shows the atomic structure of Cu5Pd nanoalloys for the electroreduction of NO3- to NH3. The H atoms adsorbed on the Pd sites prefer to transfer to adjacent nitrogen intermediates adsorbed on the Cu sites, thereby promoting the hydrogenation of intermediates and the formation of NH3. |